Cell-based screening services
“Whether you are seeking to gain insights on drug efficacy in a particular cancer type, cancer associated stromal cells or immune cells, understand mechanism of action or accelerate therapeutic development, by identifying exceptional responders, our cell based HTS pipelines can help you reach your goal.”
MISB Cancer Cell Line Collection
As an important part of our ongoing research activies, we are working to establish a comprehensive collection of new cancer model cell lines to provide the community a data and research resource to accelerate the identification and development of novel therapeutics to treat patients diagnosed with rare solid tumor types. Through collection of hundreds of patient samples, represeting 65+ different human cancers, the MISB Cancer Cell Line Collection currently consist of 60+ novel established cancer cell lines covering 40+ different solid human cancer lineages. These models have been analyzed extensively in context of Misvik’s Precision Oncology initiative in order to use the models to identify pharmacologic vulnerabilities in rare solid cancers and to identify molecular signatures that could inform novel therapeutic approaches for personalized medicine.
The MISB Cancer Cell Lines are offered for rapid large scale high-throughput panel screening assay for drug development, in-depth studies of novel drugs including combinations and synergy as well as for genome-wide loss of function genomic screening. We are continually also working to grow our cell line collection to ultimately have the largest independent and most diverse rare cancer cell line collection available.

Patient derived functional tumor models
Translational research for development of novel cancer therapies is highly dependent on representative cell-based models. However, of the currently available cancer cell lines, a large fraction of the most used models date back decades and represent only a fraction of the full complexity of human cancers limiting effective translational research and resulting in high failure rate of therapeutic agents in clinical trials. Thus, improved experimental models better reflecting human tumor biology and the genetic landscape of different cancer lineages are key to improving the success of oncology drug development. We are continuosly collecting patient derived tumor samples for translational ex vivo research in 3 ongoing clinical studies and have established a pipeline to use fresh vital tumor cell samples as functional tumor models to facilitate drug discovery and development. The collected patient derived functional tumor models enable close to clinic assessment of therapy efficacy in relevant patient populations and cancer cell lineages not represented by established cancer cell lines. These include e.g., early stage tumors with low genetic and transcriptomic background complexity and cancer cell types difficult to propagate in vitro.
The ex vivo patient derived functional tumor models also comprises samples derived from rare to extreme rare cancers for which no cell lines are described. Also matched longitudinal samples of the same patient prior and after treatment are obtained allowing study of therapy efficacy in treatment relapsed cancers. As further development of the models, the used research assays can be expanded to include also approaches for the study of the tumor immune-microenvironment, and functional analysis with cells of the tumor immune contexture for development of novel immunotherapies.
High-throughput drug screening
HTS screening of compounds or biologics with the MISB Cancer Cell Line Collection
Misvik HTS drug screening service provides you a cost effective approach to test your therapeutic compounds as single agents or as combinations.
- Rapidly assess therapy efficacy and drug responses across a broad spectrum of solid human cancer lineages
- Identify exceptional responders and resistant cancer types and correlate therapy efficacy with 150+ other cancer drugs
- Establish novel indications and stratification of cancer types for your drug candidate or drug combination
- Facilitate biomarker discovery for drug development by correlating efficacy with genetic aberrations
- Identify drug synergy with massively parallel drug combination screens
Our routine HTS service options include
- Proprietary MISB Cell Line Collection models with no third party commercial license fees (focused or pan-cancer selection of models)
- Rapid 384-well format screening with customer defined dosing, schedule and readouts
- Imaging cytometry or enzymatic analysis of therapy efficacy
- Access to a library of over 1350 FDA approved therapeutics and all available standard of care anti cancer therapeutics
- Customer defined dose matrix screens for drug combinations
Related reference publications
Ex Vivo Drug Screening Informed Targeted Therapy for Metastatic Parotid Squamous Cell Carcinoma. Nykänen N, Mäkelä R, Arjonen A, Härmä V, Lewandowski L, Snowden E, Blaesius R, Jantunen I, Kuopio T, Kononen J, Rantala JK. Front Oncol. 2021 Sep 16;11:735820. doi: 10.3389/fonc.2021.735820. PMID: 34604070
Cisplatin overcomes radiotherapy resistance in OCT4-expressing head and neck squamous cell carcinoma. Routila J, Qiao X, Weltner J, Rantala JK, et al. Oral Oncol. 2022 Apr;127:105772
Personalized Drug Sensitivity Screening for Bladder Cancer Using Conditionally Reprogrammed Patient-derived Cells. Kettunen K, Boström PJ, Lamminen T, Heinosalo T, West G, Saarinen I, Kaipio K, Rantala J, Albanese C, Poutanen M, Taimen P. Eur. J. Urol. 76(4):430-434, 2019.
Phenotypic high-throughput screening
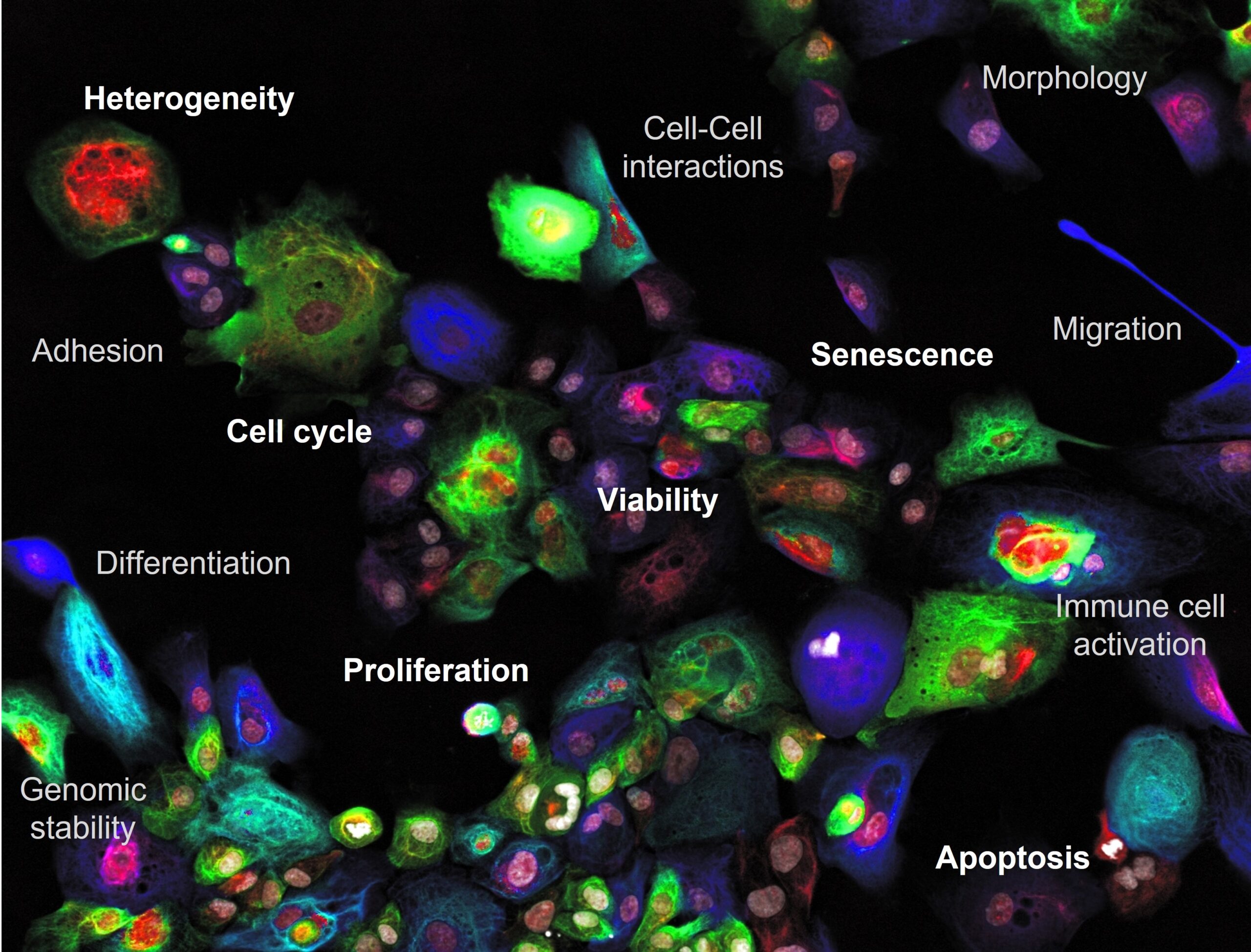
With phenotypic imaging cytometry-powered drug and RNAi screening it is possible to interrogate cellular responses at single cell resolution in comprehensive biology rich and integrated ways. Benefit from our 20-years´ experience in HTS dating back all the way to the era of invention of RNAi.
Our phenotypic HTS service options include
- Proprietary MISB Cell Line Collection models with no third party commercial license fees (focused or pan-cancer selection of models)
- Cost effective 384-well format screening with customer defined dosing, schedule and readout
- Up to genome-wide siRNA libraries, 1350 FDA approved therapeutics, hundreds of experimental cancer therapies and discovery libraries of 10 000+ diverse small molecules
- Imaging cytometry for viability and cell cycle analysis (+/- EdU incorporation)
- Immunofluorescent readouts for DNA damage (e.g., yH2Ax, 53BP1, RPA32, RAD51 foci etc.)
- Custom assays with immunofluorescent readouts for e.g., cell differentiation with lineage markers (cytokeratins and other differentiation markers)
Related reference publications
FBXL12 degrades FANCD2 to regulate replication recovery and promote cancer cell survival under conditions of replication stress. Brunner A, Li Q, Fisicaro S, Kourtesakis A, Viiliäinen J, Johansson HJ, Pandey V, Mayank AK, Lehtiö J, Wohlschlegel JA, Spruck C, Rantala JK, Orre LM, Sangfelt O. Mol Cell. 2023 Aug 9:S1097-2765(23)00599-3. doi: 10.1016/j.molcel.2023.07.026
A FBXO7/EYA2-SCFFBXW7 axis promotes AXL-mediated maintenance of mesenchymal and immune evasion phenotypes of cancer cells. Shen JZ, Qiu Z, Wu Q, Zhang G, Harris R, Sun D, Rantala J, et al. Mol Cell. 2022 Mar 17;82(6):1123-1139.e8. doi: 10.1016/j.molcel.2022.01.022. Epub 2022 Feb 18. PMID: 35182481
PTEN and DNA-PK determine sensitivity and recovery in response to WEE1 inhibition in human breast cancer. Brunner A, Suryo Rahmanto A, Johansson H, Franco M, Viiliäinen J, Gazi M, Frings O, Fredlund E, Spruck C, Lehtiö J, Rantala JK, Larsson LG, Sangfelt O. Elife. 2020;9:e57894